
Recently, Jiangsu Hansoh Pharmaceutical Group Co., Ltd. ("Hansoh Pharma" or "Company") received the Notice of Approval for Clinical Trial of Drug from the National Medical Products Administration ("NMPA"), according to which the Company is approved to carry out clinical trial of HS-10365 Capsules, a Class 1 innovative drug with independent intellectual property rights, for advanced malignant solid tumors.
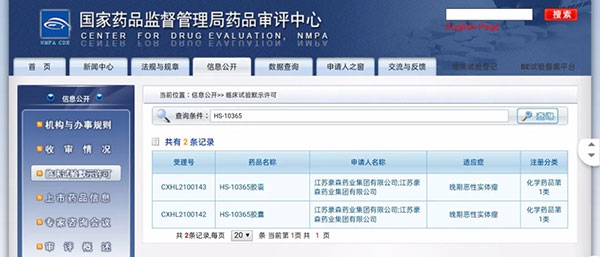
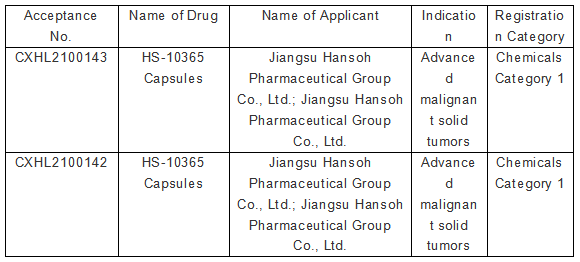
▲ Screenshot of public information from CDE, NMPA
